 |
| (bioquicknews.com) |
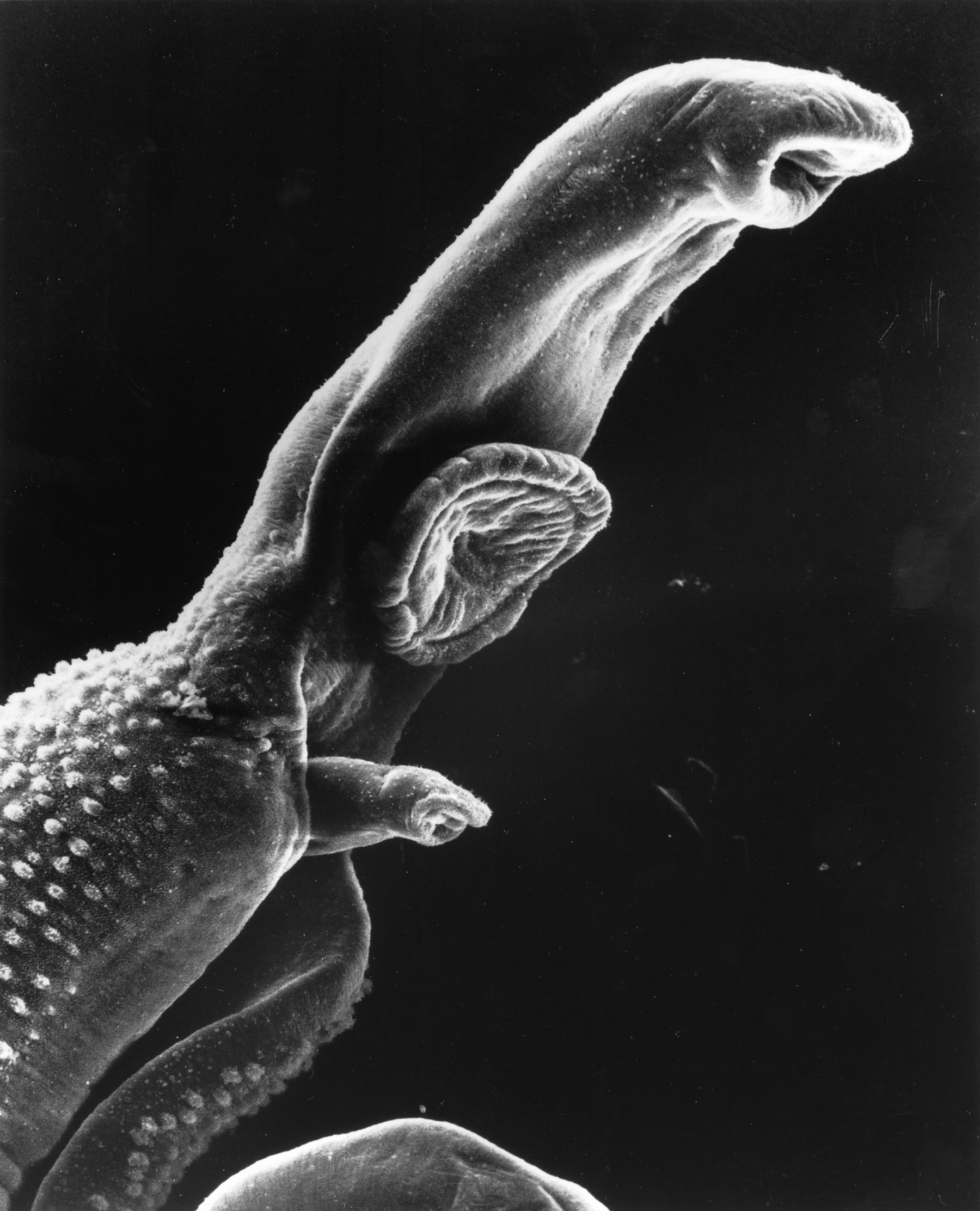
Schistosoma mansoni (left) is a very interesting worm. In fact, it is one of the "Old Friends" with which we have co-evolved. This parasite is the responsible for the development of schistosomiasis, to which no vaccine has been developed yet.
What seems most interesting about S.mansoni is its capacity to modulate the human immune system. While in the host, each adult worm pair releases 200-300 eggs each day, which maturate during 5-6 days. Maturation involves forming the Von Lichtenberg's envelope on the inside of the egg shell from which proteins are secreted. The host reacts to the eggs and the proteins secreted, mounting a Th2-type immune response. One group of proteins present in the egg are soluble egg antigens (SEA). The exact mechanism by which SEA and other proteins secreted affect the immune response is not entirely clear. Nevertheless, it seems to involve C-type lectin receptors (CLR), as most antigenic proteins identified so far are glycoproteins with some conserved glycans (1).
One characteristic of parasitic helminths like S. mansoni is that they have developed many strategies to supress the host response, thereby controlling the inflammatory response. This has been a mechanism acquired through co-evolution with its mammalian hosts, like humans. As parasite driven infection induces a Th2-type immune response, it can be hypothesized that some parasites can modulate the onset and progression of Th1-type autoimmune diseases, like type I diabetes.
NOD mice is a model for autoimmune type I diabetes. When infected with S.mansoni or exposed to SEA, these mice do not develop diabetes (2). To try to elucidate the mechanism, Zaccone et al (3) examined the effect of SEA on Foxp3+ Treg levels. Now, Treg cells are very important for controlling the immune response (hence the name Treg-regulatory T cells). One of the most important roles of these cells is establishing self-tolerance. Any failure of self tolerance would potentially lead to the development of autoimmunity. What has been observed is that injection of SEA to NOD mice increases the population of CD4+Foxp3+T cells in the pancreas. SEA generates Foxp3+ T cells from naïve CD4 T cells in a TGF-b dependent fashion. This effect was shown to happen directly and indirectly (by upregulation of CLR on dendritic cells), which suggests a synergistic response. Specific CLR upregulated by SEA include galectins 1 and 3, SIGN-R1 and DEC-205. Moreover, SEA is capable to alternatively activate macrophages (4), consistent with the effect of other helminths (5). Depletion of CD25+ T cells from splenocytes of SEA-treated NOD mice restored their ability to transfer diabetes to recipient animals, supporting the role of Treg in diabetes prevention in SEA-treated NOD mice. In contrast, depletion of CD25+ T cells from splenocytes of serum worm antigen (SWA)-treated NOD mice did not restore the ability to transfer diabetes to recipient NOD mice. This suggests that SEA and SWA protect against diabetes by different mechanisms, and that protection from SEA is mediated primarily by Tregs.
The protective effect of S.mansoni on other Th-1 type autoimmune diseases has also been observed. For instance, helminth infection seems to protect against the course of multiple sclerosis (MS) (6). Although this study did not correlate infection with S.mansoni and the severity of MS exclusively, animal models of experimental autoimmune encephalitis (EAE) (an animal model for MS) infected with S.mansoni exhibit reduced levels of proinflammatory cytokines and CNS inflammation (7, 8). Exposure to S.mansoni eggs and/or infection also protects mice from TBNS-induced colitis (animal model for Chron's disease) (9, 10), collagen-induced arthritis (animal model for rheumatoid arthritis) (11), Grave's disease (12), and allergic diseases (13, 14). This challenges the Th1/Th2 dichotomy and suggests that there are other mechanisms by which S.mansoni modulates the immune system.
The rise in inflammatory and autoimmune conditions may not only have a nutritional basis. Lack of exposure to some essential parasites for the development of the immune system by adoption of extreme hygiene practices during early infancy may increase the risk of allergic and autoimmune diseases. A healthy lifestyle should not only include avoiding industrialized foods, but also being exposed to different pathogens which help shape our immune system.
Interesting. It is quite true. Microorganisms are have long played a role in the development of vaccines and anti-toxins. Without them it wouldn't be possible for the human body to develop the defense against them.
ReplyDelete